Water purification systems vs. bottled pure water
How an in-house water purification system can improve results, save money and cut waste

Water’s the most common reagent in the lab, used for everything from preparing samples to rinsing instruments. But it must be exactly the right purity to avoid invalidating your results. That’s particularly the case for highly sensitive analyses, like high-performance liquid chromatography (HPLC) and ion chromatography, where even minor contamination can have significant consequences.
So what’s the best way to get the right grade of water your lab needs for its applications?
Watch our video and then read on to discover more...
Bottled pure water: more challenging than it first seems
At first glance, bottled pure water can seem the most simple and economical solution. After all, there’s no capital investment or ongoing maintenance to consider. But in reality there are many pitfalls:
1) Contamination from airborne pollutants
Bottled Ultrapure water (Type 1) degrades to general grade water (Type 3) within just 5 minutes of opening.
That’s because it quickly absorbs CO 2 and other pollutants from the air, reducing resistivity from around 18.2 MΩ-cm to 0.05 MΩ-cm. Any partially used bottles can’t be kept for later and must be thrown away. [See Figure 1]
2) Contamination from packaging leachables
It can be financially tempting to buy bottled pure water in large volumes and store it for future use. But over time leachables from bottles, like solvents and inorganic ions, can contaminate the water. That means the stated purity offers no guarantee of the actual purity upon opening.
3) High and escalating costs
As bottled pure water is so easily contaminated, you’ll end up with a lot of wasted water if you buy large quantities. Purchasing smaller volumes can help overcome this issue, but this can be very expensive and stretch your lab’s budget.
Find out more about the problems with using bottled pure water.
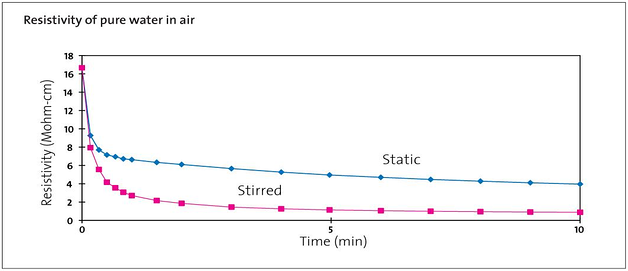
[Figure 1] Resistivity of pure water in air - Sourced from Elga Veolia
Water purification systems: the smarter alternative
1) Increase the validity of your results
Water purification systems turn simple tap water into Ultrapure water. But the great advantage is that they constantly monitor and maintain purity levels, giving you total confidence in your lab’s water and analytical accuracy at all times.
The better option is to invest in an in-house water purification system. Here are three reasons why:
2) Save on your lab’s running costs
Even accounting for initial investment and maintenance, water purification systems offer very low costs per litre over the long term. In fact, when using volumes of 10 litres a day, the cost per litre of in-house pure water could be as little as £1 – compared to as much as £55 for bottled pure water. That means the savings on your lab’s running costs could be huge.
3) Cut down on waste
Water purification systems allow you to dispense the exact amount of water you need, when you need it. So compared to bottled water, your lab will produce far less waste and help reduce the world’s plastic pollution problem. Not only that, but you won’t have storage issues to consider.
Discover how our innovative Purelab Chorus range of water purification systems could improve your lab’s results and slash running costs.





